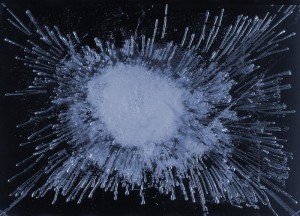
Ice image of distilled water: A sparse and ordered structure surrounds the core. Photo: From the Gisela Ahlberg collection, Institute of Ecological Technology. (The photos have been slightly colorized.)
The temperature drops, water cools, and suddenly, there is a crest of ice on the surface, slowly growing and deepening. Soon it will be so hard that we can touch it without destroying it, and even walk on it. Anyone who has been skating on a lake will know that there is ice and there is ice. Sometimes clear, sometimes so filled with air bubbles as to be almost completely opaque.
The Swedish water investigator Gisela Ahlberg decided to explore this, and devised a method, together with her colleague Christina Weldero, to study natural waters by ice images.

Ice image of well water from Nacka spring, outside Stockholm: An ordered structure of air bubbles at the periphery. Photo: From the Gisela Ahlberg collection, Institute of Ecological Technology.

Tap water from Stockholm: The frozen air bubbles lack directional structure. Photo: From the Gisela Ahlberg collection, Institute of Ecological Technology.

Vortexed tap water: The ice bubbles show more distinct structure, resembling the spring water. Photo: From the Gisela Ahlberg collection, Institute of Ecological Technology.

A bottled mineral water: Its structure a mix of the tap water and the spring water. Photo: From the Gisela Ahlberg collection, Institute of Ecological Technology.
And clearly, water samples from different sources show distinctly different patterns. When water freezes, air bubbles are released, and these arrange themselves in various patterns, depending on how the crystallization process of the water proceeds.
Subjecting water to vortexing would alter the typical pattern of the image. A factor is of course the content of air dissolved in water – but also how the air is bound into the water. That, is probably one of the most overlooked properties of water.
This process has indeed been applied in large scale by the Swedish engineer Curt Hallberg and his colleagues. Treating water on an ice rink by vortexing it vigorously will affect the subsequent freezing process. The result is a different ice, an effect that can easily be observed by a professional skater. The ice is experienced as less brittle, and faster. Somehow, the effect of vortexing has carried over into the solid phase.
Read more
Much of Gisela Ahlberg’s documentation on ice images still remains unpublished. I have given a brief overview of the ice image method and alternative ways to measure water quality in the following report. See particularly chapter 3.2:
-
Johansson L. Alternativ vattenbehandling – Effekter, mekanismer och perspektiv på vattenkvalitet Institute of Ecological Technology, Scientific & Technical Reports, No. 2, Gothenburg, 2005, ISBN 91-975722-1-7 (downloadable)
The image of vortexed water shows Stockholm tap water after it has passed through a “Martinwirbler”, a vortexing device developed by Wilhelm Martin in co-operation with Walter Schauberger. Read more about it here:
- Martin vortexer (in German)
More information about the vortexing process developed by Curt Hallberg and his colleagues at Watreco in Sweden can be found here:
- Watreco Vortex Process Technology overview.
- Some technical details on the vortex forming process, and a demonstration of vortex degassing of water.
- Realice – an application of the vortex technology that changes the structure of the ice at ice rinks, and as a side effect reduces the energy consumption.